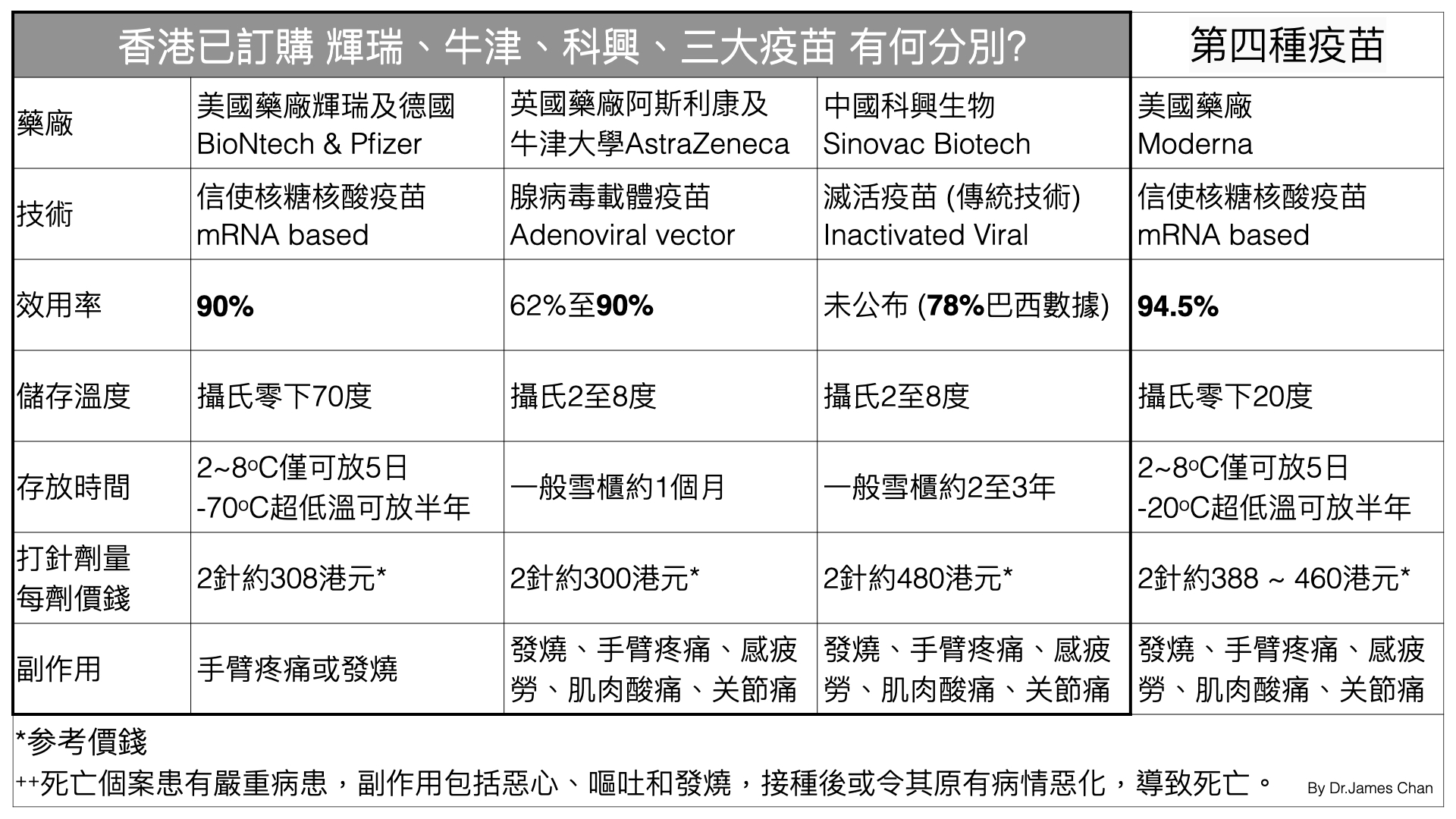
Moderna reported that during the phase 3 study of its vaccine candidate mRNA-1273, which enrolled 30,000 adult U.S. participants, just five of the 95 COVID-19 cases occurred among the vaccinated, while 90 infections were identified in the placebo group. This corresponds to an efficacy of 94.5%. None of the infected patients who received the vaccine developed severe COVID-19, while 11 (12%) of those who received the placebo did.
Similarly, the Pfizer-BioNTech vaccine candidate, BNT162b2, was 90% effective in preventing infection during the phase 3 clinical trial, which enrolled 43,538 participants, with 30% in U.S. and 42% abroad.